Jan 24, 2024
Discovery of a protein supporting numerous functions of cell nuclei
—Understanding laminopathy that causes muscular dystrophy—
Keyword:RESEARCH
OBJECTIVE.
A team of researchers has discovered that in Drosophila, the uniform mesh structure of the nuclear lamina of cell nuclei (Fig. 1A) supports a variety of nuclear functions. The team members include Professor Satoshi Goto, Specific Project Research Fellow Miki Yamamoto (Hino), postdoctoral researcher Kohei Kawaguchi (now a specially appointed assistant professor at Tokyo Institute of Technology) of the Rikkyo University College of Science; Associate Professor Yuka Iwasaki of the Department of Molecular Biology at the Keio University School of Medicine (now a team leader at RIKEN); and Masahito Tanaka and Yuta Shimamoto, researcher and associate professor, respectively, with the Department of Chromosome Science at the National Institute of Genetics.
These findings are expected to contribute to our understanding of disease. That is, while it is known that laminopathy, a disease caused by mutation of the nuclear lamina, causes muscular dystrophy-like symptoms in humans, muscle tearing was also observed in Drosophila with abnormalities in the uniformity of the nuclear lamina, which led to muscular dystrophy-like symptoms. These findings will be of great help in trying to understand the causes of laminopathy that leads to muscular dystrophy. The study was published online in the American Journal of Cell Biology on January 23, 2024.
Background
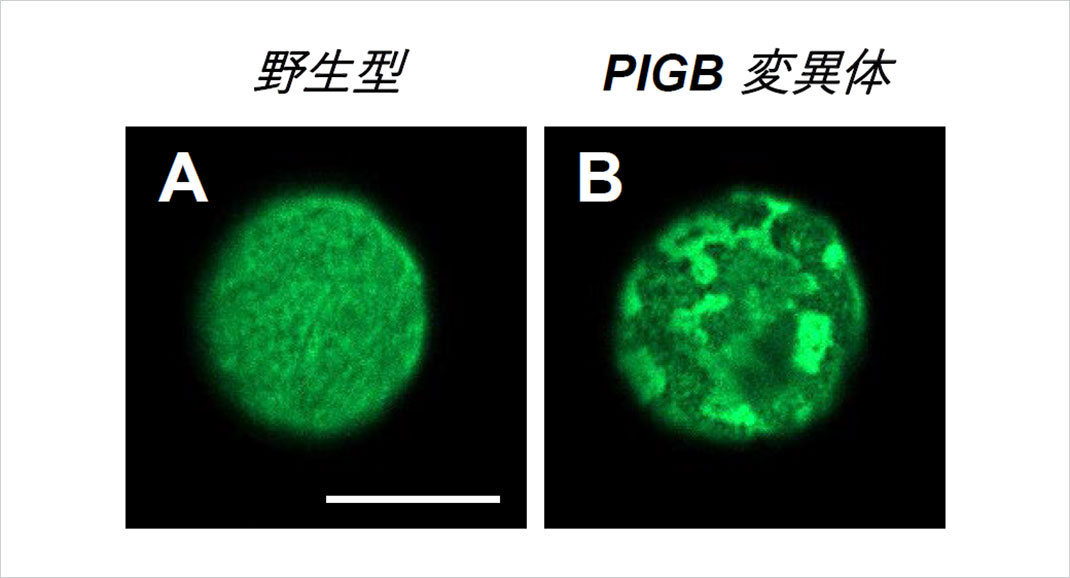
Figure 1. A uniform nuclear lamina could be seen in the wild type (A), while the PIGB mutant exhibited a disturbed and irregular lamina (B). Bar, 10μm.

Figure 2. Nuclear envelope proteins (nuclear pore complex, A) were distributed uniformly in the wild type, but showed an irregular pattern in the PIGB mutant (B).
Findings
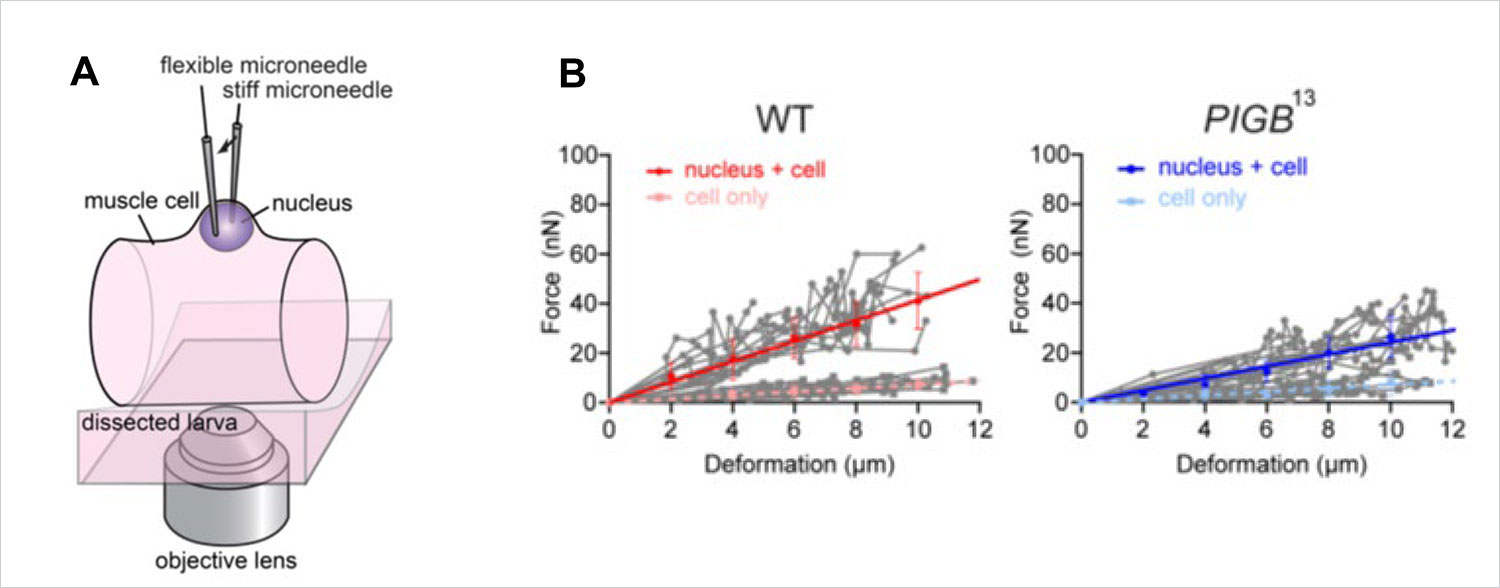
Figure 3. (A) The nucleus was held between two glass needles, and the strength of the nucleus was calculated by measuring the amount of force applied and the degree of deformation of the nucleus. (B) The steeper the solid line, the harder the nucleus. The nucleus of the PIGB mutant was softer than that of the wild type (WT).
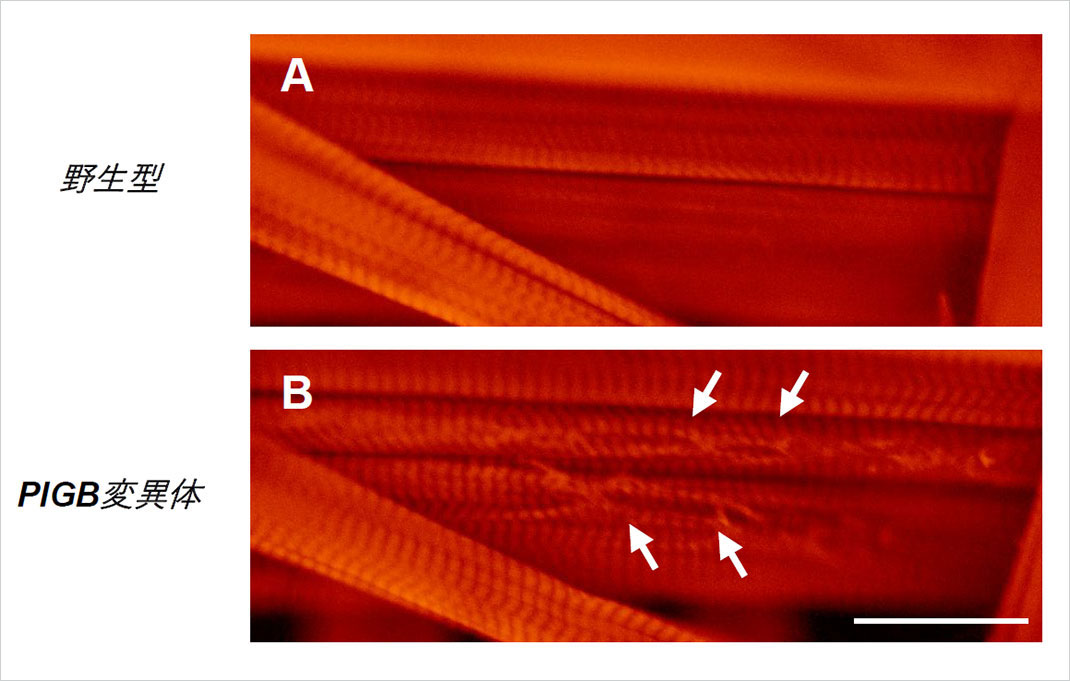
Figure 4. Muscles of a Drosophila larva. The wild type (A) exhibited orderly muscle tissue, while the PIGB mutant (B) had tears in the muscle tissue (arrow).
Summary and future prospective
Article information
- Journal: Journal of Cell Biology
- DOI: 10.1083/jcb.202301062
- Paper title: PIGB maintains nuclear lamina organization in skeletal muscle of Drosophila
- Authors: Miki Yamamoto-Hino, Masaru Ariura, Masahito Tanaka, Yuka W. Iwasaki, Kohei Kawaguchi, Yuta Shimamoto, Satoshi Goto