Mar 24, 2023PRESS RELEASE
Developing CO2 conversion method without using rare metals
- High expectations for achieving carbon neutrality -
Keyword:RESEARCH
OBJECTIVE.
A joint research team of scientists from Kobe University and Rikkyo University has developed a method to produce formic acid*2 from carbon dioxide (CO2) without using rare metals*1.
The team is led by Associate Professor Ryosuke Matsubara of the Graduate School of Science and Professor Yasuhiro Kobori of the Molecular Photoscience Research Center, Kobe University; and Professor Masahiro Yamanaka of the College of Science, Rikkyo University.
The increase in the atmospheric concentration of CO2, a gas that causes global warming, is a major social issue. And the fossil fuels (oil, natural gas, etc.) that power human society will inevitably be depleted in the future. To solve these two challenging problems, a process called artificial photosynthesis, which uses sunlight to chemically convert CO2 into fossil fuels or their equivalents, has gained increasing attention as a way to possibly kill two birds with one stone. It is currently being studied in various countries. Most methods of artificial photosynthesis, however, require the use of rare metals. This has been a major obstacle preventing the introduction of artificial photosynthesis on a global scale.
In this study, Associate Professor Matsubara, Professor Kobori, and Professor Yamanaka created a new catalyst system*3 that does not use rare metals, and developed a photochemical reaction that can convert CO2 to formic acid under mild conditions, that is, room temperature and CO2 at one atmosphere. This reaction does not require the injection of electrical energy from an external energy source. Adequate sunlight is sufficient to start the reaction.
In future, the team plans to develop a catalyst system that does not require the use of a sacrificial reductant*4, which was necessary for the reaction in this study, and reactions that produce carbon fuels other than formic acid, such as methane and methanol.
The results of this study will be published in the online version of Nature Chemistry by Britain’s Nature Publishing Group at 4 p.m. on Thursday, March 23, 2023, British time (Japan time: 1 a.m., March 24).
The increase in the atmospheric concentration of CO2, a gas that causes global warming, is a major social issue. And the fossil fuels (oil, natural gas, etc.) that power human society will inevitably be depleted in the future. To solve these two challenging problems, a process called artificial photosynthesis, which uses sunlight to chemically convert CO2 into fossil fuels or their equivalents, has gained increasing attention as a way to possibly kill two birds with one stone. It is currently being studied in various countries. Most methods of artificial photosynthesis, however, require the use of rare metals. This has been a major obstacle preventing the introduction of artificial photosynthesis on a global scale.
In this study, Associate Professor Matsubara, Professor Kobori, and Professor Yamanaka created a new catalyst system*3 that does not use rare metals, and developed a photochemical reaction that can convert CO2 to formic acid under mild conditions, that is, room temperature and CO2 at one atmosphere. This reaction does not require the injection of electrical energy from an external energy source. Adequate sunlight is sufficient to start the reaction.
In future, the team plans to develop a catalyst system that does not require the use of a sacrificial reductant*4, which was necessary for the reaction in this study, and reactions that produce carbon fuels other than formic acid, such as methane and methanol.
The results of this study will be published in the online version of Nature Chemistry by Britain’s Nature Publishing Group at 4 p.m. on Thursday, March 23, 2023, British time (Japan time: 1 a.m., March 24).
Key points of the study
- The team has developed a photochemical reaction that converts CO2 to formic acid without using rare metals.
- Expectations are high for the practical use of this reaction, which reduces greenhouse gas emissions and forms fossil fuels at the same time.
Background and history of the study
The enzymes involved in plant photosynthesis use sunlight to convert CO2 into sugar (glucose). Thanks to photosynthesis, the Earth has a stable atmospheric environment. But human activities such as CO2 emissions and fossil fuel consumption have now outstripped the homeostatic effects of plants. Developing artificial photosynthesis is thus considered essential for humanity’s survival, and is now being studied worldwide. In the course of such studies, it has been found that metallic elements, especially rare metals, have high catalytic activity. But because they are rare, their widespread, practical use on a global scale presents challenges.
Associate Professor Matsubara and other scientists have been studying metal-free photosensitizers(*5) that have a high reducing capability (the ability to donate electrons to other molecules). Recently, through molecular structure modification, we have developed a metal-free photosensitizer that can acquire a high reducing capability under the irradiation of visible light, even though it has lower energy than ultraviolet light. This has made it possible to realize photoreaction that can directly utilize sunlight, which is mainly composed of visible light components.
In this study, photoreduction of CO2 under visible light irradiation has been achieved by using yet another metal-free catalyst along with the photosensitizer.
Associate Professor Matsubara and other scientists have been studying metal-free photosensitizers(*5) that have a high reducing capability (the ability to donate electrons to other molecules). Recently, through molecular structure modification, we have developed a metal-free photosensitizer that can acquire a high reducing capability under the irradiation of visible light, even though it has lower energy than ultraviolet light. This has made it possible to realize photoreaction that can directly utilize sunlight, which is mainly composed of visible light components.
In this study, photoreduction of CO2 under visible light irradiation has been achieved by using yet another metal-free catalyst along with the photosensitizer.
Content of the study
Construction of catalyst system: In order to convert CO2 into formic acid, it is necessary to donate electrons to CO2, that is, a CO2 reduction reaction. However, CO2 is a very stable molecule and is not easy to reduce. In this study, the team developed carbazole 1 as a photosensitizer and verified that its molecules have a significantly high reducing capability under visible light irradiation (Figure 1). Although the efficiency of CO2 photoreduction reaction (formic acid formation) was low with this photosensitizer alone, we have discovered that the addition of a metal-free catalyst 2 dramatically increases the formic acid formation rate.
The team has confirmed that under optimal conditions, as many as 6,500 or more formic acid molecules can be produced from a single carbazole 1 molecule, indicating that an excellent catalyst system has been constructed.
Explanation of the reaction mechanism: We tried to find an explanation for the mechanism of the developed CO2 photoreduction reaction.
(1) The team conducted an experiment performing a reaction using CO2 labeled with 13C*6. As a result, formic acid labeled with 13C was generated. This proves that formic acid is formed from CO2.
(2) The team used time-resolved spectroscopy*7 to clarify the electron transfer process after carbazole 1 absorbs light (Figure 2a). As a result, we discovered that carbazole 1, after absorbing light, transfers electrons respectively to precursor 3 of catalyst 2 (Figure 1) and CO2 on a time scale of nanoseconds to microseconds (nanosecond: one billionth of a second; microsecond: one millionth of a second).
(3) After receiving electrons from carbazole 1, CO2 is converted to CO2 radical anion (a chemical species). The team analyzed by computer calculation the subsequent conversion of the CO2 radical anion to formic acid (Figure 2b). As a result, it has verified that the CO2 radical anion and catalyst 2 conduct hydrogen atom transfer to produce formic acid.
Based on discussions concerning the above, the team has proposed a reaction mechanism for this reaction.
The team has confirmed that under optimal conditions, as many as 6,500 or more formic acid molecules can be produced from a single carbazole 1 molecule, indicating that an excellent catalyst system has been constructed.
Explanation of the reaction mechanism: We tried to find an explanation for the mechanism of the developed CO2 photoreduction reaction.
(1) The team conducted an experiment performing a reaction using CO2 labeled with 13C*6. As a result, formic acid labeled with 13C was generated. This proves that formic acid is formed from CO2.
(2) The team used time-resolved spectroscopy*7 to clarify the electron transfer process after carbazole 1 absorbs light (Figure 2a). As a result, we discovered that carbazole 1, after absorbing light, transfers electrons respectively to precursor 3 of catalyst 2 (Figure 1) and CO2 on a time scale of nanoseconds to microseconds (nanosecond: one billionth of a second; microsecond: one millionth of a second).
(3) After receiving electrons from carbazole 1, CO2 is converted to CO2 radical anion (a chemical species). The team analyzed by computer calculation the subsequent conversion of the CO2 radical anion to formic acid (Figure 2b). As a result, it has verified that the CO2 radical anion and catalyst 2 conduct hydrogen atom transfer to produce formic acid.
Based on discussions concerning the above, the team has proposed a reaction mechanism for this reaction.
Future prospects
The reaction developed in this study requires vitamin C (ascorbic acid) as a sacrificial reductant. There is room to improve in this area. In the future, the team plans to develop a reaction using water as a reductant instead of ascorbic acid. It also aims to develop a CO2 photoreduction reaction to produce methane and methanol as energy materials in addition to formic acid.
Reference materials
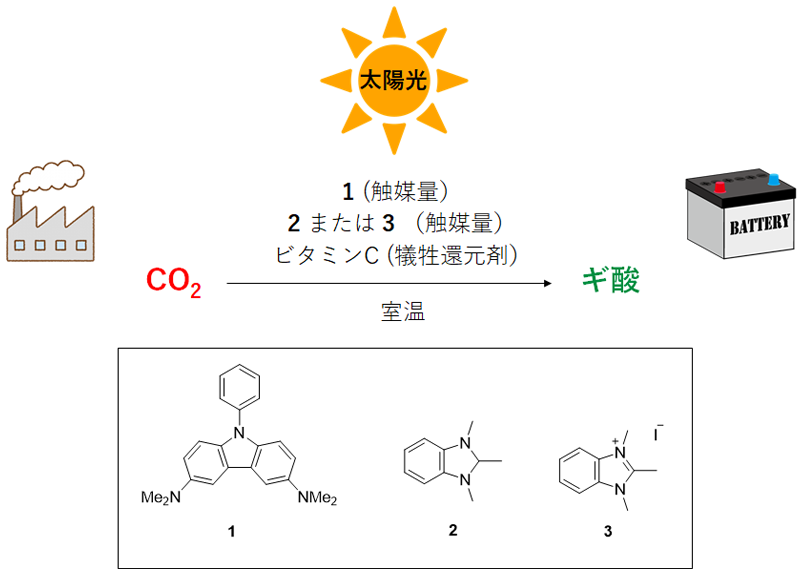
Figure 1: Formic acid production by CO2 photoreduction reaction without using rare metals
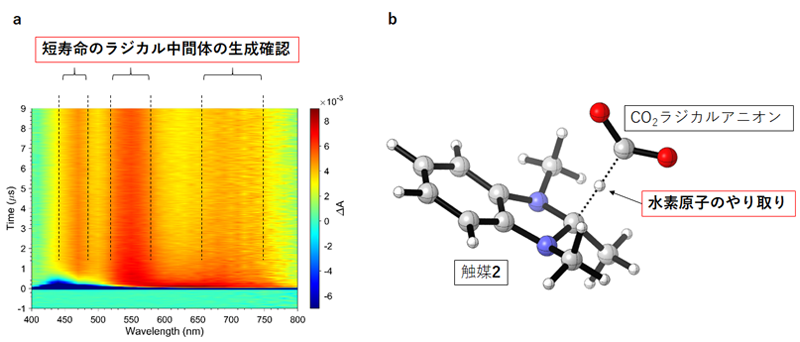
Figure 2. Analysis of reaction mechanism experiment
(a) Detection of short-lived chemical species by time-resolved spectroscopy. The team conducted reaction tracking on the order of one millionth of a second using a special analyzer and as a result, observed the predicted signals originating from short-lived intermediates.
(b) Computational chemistry analysis of molecular structure during the reaction. The team analyzed the reaction process, which is difficult to observe, by computation performed by a computer, and confirmed the reaction format that we predicted is valid.
(b) Computational chemistry analysis of molecular structure during the reaction. The team analyzed the reaction process, which is difficult to observe, by computation performed by a computer, and confirmed the reaction format that we predicted is valid.
Glossary
*1 Rare metals: Metallic elements that can be mined only in small quantities. They often show high catalytic activity in various reactions, but they are expensive, and supplies tend to be unstable due to social conditions.
*2 Formic acid: A carboxylic acid with the molecular formula HCOOH. It has attracted attention in recent years as one of the energy resources for fuel cell materials, along with hydrogen and methanol.
*3 Catalyst system: A catalyst is a substance that accelerates a reaction without changing its own form before and after the reaction. As a catalyst does not change its own form, it is possible to produce a much larger quantity of the target substance than its own weight. Catalysts play a key role in the modern chemical industry. A catalyst system is the entire condition of catalysis, including additives, solvents, and reaction temperatures in addition to the catalyst involved.
*4 Sacrificial reductant: A reductant is a substance that functions as a source of electrons in reduction reaction. In energy production processes such as hydrogen production and CO2 reduction reaction, the most ideal reductant is water. When direct use of water is difficult, an alternative reductant is used. A reductant used in this way is called a sacrificial reductant.
*5 Photosensitizer: A substance that can absorb light and utilize the obtained light energy for chemical reaction. The term is sometimes used in a way that is nearly equivalent to “photocatalyst.”
*6 13C: One of the stable isotopes of carbon. Of all natural carbon atoms on the Earth, 99% are 12C, which has six protons and six neutrons in its nucleus, and only 1% are 13C, which has one more neutron than 12C. For this reason, when investigating the origin of carbon atoms in a substance, an experimental method is often used in which a reaction is conducted using molecules with an artificially increased ratio of 13C atoms (called 13C-labeled molecules) compared to their natural ratio, for the purpose of tracking the 13C atoms.
*7 Time-resolved spectroscopy: Spectroscopy is a method to analyze the properties and quantities of substances by observing light absorption and light emission per wavelength. Time-resolved spectroscopy is an analytical method in which spectroscopy is performed continuously for every elapsed time from a certain point in time (the point of light irradiation, for example), allowing analysis in very short time units such as one billionth of a second.
*2 Formic acid: A carboxylic acid with the molecular formula HCOOH. It has attracted attention in recent years as one of the energy resources for fuel cell materials, along with hydrogen and methanol.
*3 Catalyst system: A catalyst is a substance that accelerates a reaction without changing its own form before and after the reaction. As a catalyst does not change its own form, it is possible to produce a much larger quantity of the target substance than its own weight. Catalysts play a key role in the modern chemical industry. A catalyst system is the entire condition of catalysis, including additives, solvents, and reaction temperatures in addition to the catalyst involved.
*4 Sacrificial reductant: A reductant is a substance that functions as a source of electrons in reduction reaction. In energy production processes such as hydrogen production and CO2 reduction reaction, the most ideal reductant is water. When direct use of water is difficult, an alternative reductant is used. A reductant used in this way is called a sacrificial reductant.
*5 Photosensitizer: A substance that can absorb light and utilize the obtained light energy for chemical reaction. The term is sometimes used in a way that is nearly equivalent to “photocatalyst.”
*6 13C: One of the stable isotopes of carbon. Of all natural carbon atoms on the Earth, 99% are 12C, which has six protons and six neutrons in its nucleus, and only 1% are 13C, which has one more neutron than 12C. For this reason, when investigating the origin of carbon atoms in a substance, an experimental method is often used in which a reaction is conducted using molecules with an artificially increased ratio of 13C atoms (called 13C-labeled molecules) compared to their natural ratio, for the purpose of tracking the 13C atoms.
*7 Time-resolved spectroscopy: Spectroscopy is a method to analyze the properties and quantities of substances by observing light absorption and light emission per wavelength. Time-resolved spectroscopy is an analytical method in which spectroscopy is performed continuously for every elapsed time from a certain point in time (the point of light irradiation, for example), allowing analysis in very short time units such as one billionth of a second.
Acknowledgments
This work was supported by the following grants:
(Matsubara) ENEOS TonenGeneral Research/Development Encouragement & Scholarship Foundation, Takahashi Industrial and Economic Research Foundation, The Takano Science Foundation, Fukuoka Naohiko Memorial Foundation, and Kanamori Foundation
(Kobori) JSPS grants-in-aid for Transformative Research Areas (A), “Dynamic Exciton” (JP20H05832, JP20K21174, JP20KK0120, JP22K19008)
(Matsubara) ENEOS TonenGeneral Research/Development Encouragement & Scholarship Foundation, Takahashi Industrial and Economic Research Foundation, The Takano Science Foundation, Fukuoka Naohiko Memorial Foundation, and Kanamori Foundation
(Kobori) JSPS grants-in-aid for Transformative Research Areas (A), “Dynamic Exciton” (JP20H05832, JP20K21174, JP20KK0120, JP22K19008)
Article Information
- Title:
“Metal-free reduction of CO2 to formate using a photochemical organohydride-catalyst recycling strategy”
DOI: 10.1038/s41557-023-01157-6
- Authors:
Weibin Xie, Jiasheng Xu, Ubaidah Md Idros, Jouji Katsuhira, Masaaki Fuki, Masahiko Hayashi, Masahiro Yamanaka, Yasuhiro Kobori, and Ryosuke Matsubara
- Journal:
Nature Chemistry