Jul 09, 2020PRESS RELEASE
Development of a novel porous material with selective CO2 adsorption
Keyword:RESEARCH
OBJECTIVE.
A research group at the Mirai Theme Project Laboratory (Smart Molecules Laboratory) that included Rikkyo University Prof. Mao Minoura, Assistant Prof. Koh Sugamata, and Visiting Prof. Teruyuki Iihama of the Department of Chemistry, as part of efforts to create environmentally friendly molecules, developed a novel material that selectively adsorbs the greenhouse gas, CO2 (Figure 1). The laboratory is an endowed Rikkyo University research project* funded by Nippon Soda Co.** This material can also adsorb hydrogen molecules, which are known to be difficult to handle, and could thus be used as a kind of "Molecular Cylinder" for storing hydrogen in fuel-cell vehicles. This is a significant achievement of industry-academia collaboration between Nippon Soda and Rikkyo University that received the high honor of being published in the Royal Chemistry Society's journal "Dalton Transactions."
Since 2017, Rikkyo University has been pursuing research projects endowed by companies and other entities with the goal of promoting the development and vitalization of research and education, and contributing to society through technological collaboration. The hope is that endowed research projects will generate the collaborative effects associated with industry-academia partnerships.
**) Nippon Soda Co.
A comprehensive chemical company that celebrated its 100th anniversary in 2020. Akira Ishii is its president. Under a new slogan—"Shining with chemistry"—the company manufactures and markets high value-added chemical products such as agricultural chemicals, pharmaceutical additives, and electronic materials.
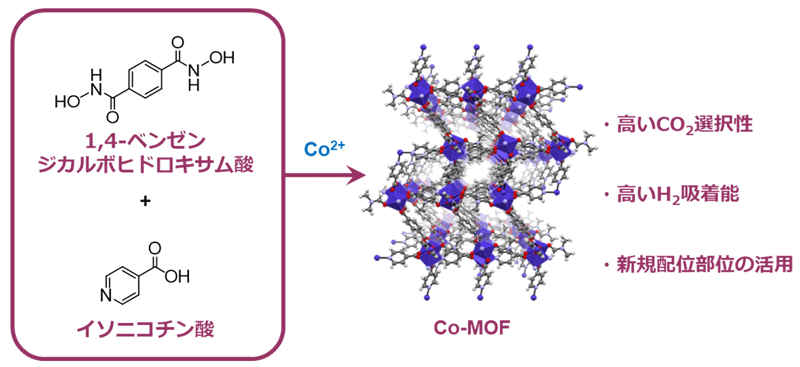
Figure 1: Molecular structure illustration of the new metal-organic framework (MOF) developed in this study
1. Key points of the study's achievements
2) In addition to selective adsorption of CO2, the material can store hydrogen gas, which is a promising clean energy source.
3) The research group succeeded in using hydroxamate groups, which have rarely been used to construct MOF.
Metal-organic frameworks*1) possess extremely small pores called micropores*2) with specific surface area that far exceeds those of conventional porous materials such as activated carbon and zeolite*3). It is expected to be applied to gas adsorption and separation are anticipated. By using benzene-1,4-dicarbohydroxamic acid as an organic ligand and isonicotinic acid as an auxiliary ligand, and reacting with cobalt nitrate, the research group succeeded in developing an MOF that can selectively adsorb carbon dioxide and store large amounts of hydrogen (Figure 1).
In addition, a hydroxamate (RCONHO-), which has rarely been used in MOF synthesis, was used as the coordination site, which raises hopes for future applications of this novel coordination site in MOF.
*1) Metal-organic framework (MOF): A general term for coordination polymers composed of organic ligands and metal ions. Also known as organometallic structures.
*2) Micropores: Tiny pores in porous materials with a diameter of 2 nm or less. Pores that are 2-50 nm are called a mesopores, and those 50 nm or larger are called macropores.
*3) Zeolite: A generic term for crystalline aluminosilicates. Discovered as a natural mineral, they are now synthesized to have various pore sizes and shapes.
2. Background of the study
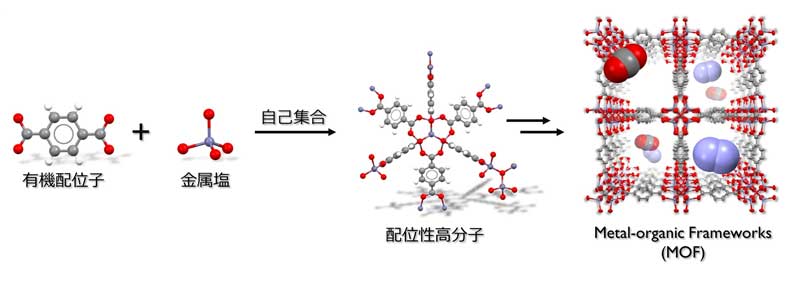
Figure 2: Synthesis and structure of metal-organic frameworks
3. Results of the study
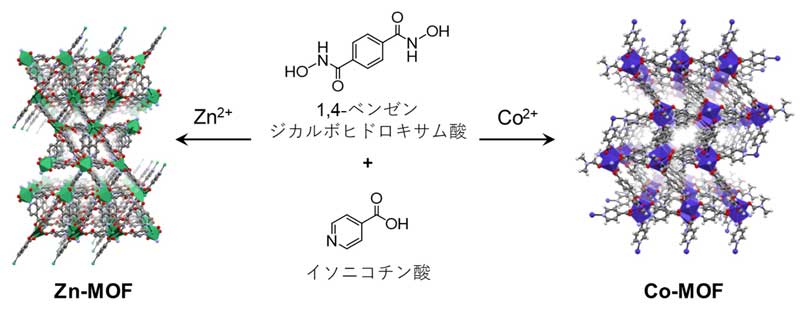
Figure 3: Zn-MOF (left) and Co-MOF (right) with hydroxamate sites
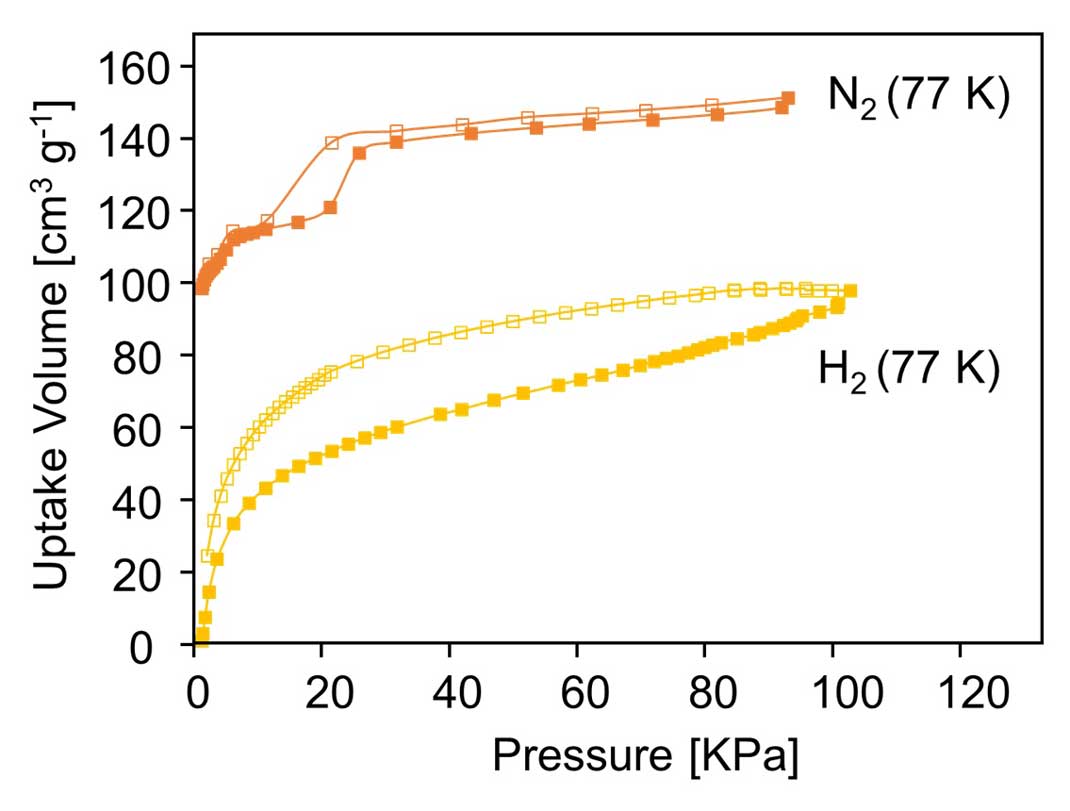
Figure 4: Nitrogen and hydrogen adsorption isotherm at 77 K
Next, the research group investigated the selectivity of the material's gas adsorption. First, the amount of nitrogen and CO2 adsorption was measured at room temperature. To predict the selectivity for a CO2/N2 binary mixture, ideal adsorbed solution theory (IAST) calculations, coupled with a dual-site Langmuir-Freundlich simulation, were employed on the basis of single-component isotherms. Figure 5 shows the predicated selectivity for CO2/N2 as a function of the pressure when the gas phase mole fraction is 15/85, which is a typical feed composition of flue gas (Figure 5). This showed that the MOF selectively adsorbed 39 times more CO2 than nitrogen at room temperature (Figure 6). This ranks among the best ever reported for selectivity of an MOF, and raises hopes for future applications to CO2 separation membranes.
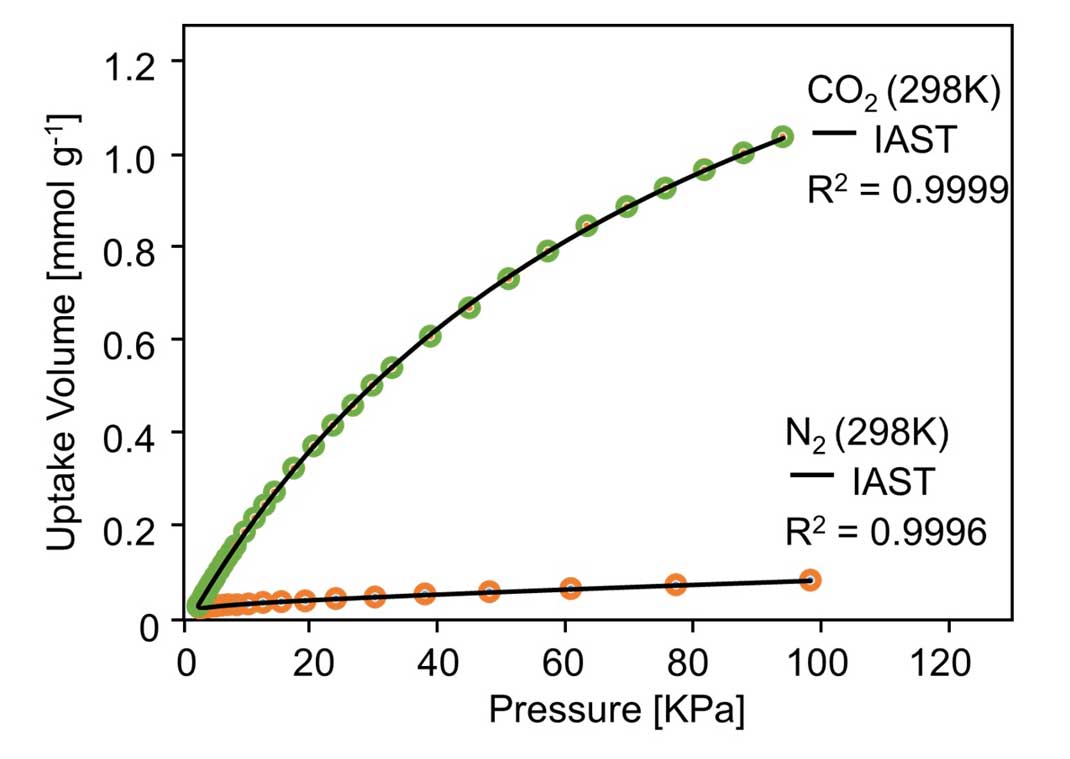
Figure 5: Nitrogen and CO2 adsorption with IAST fitting at room temperature
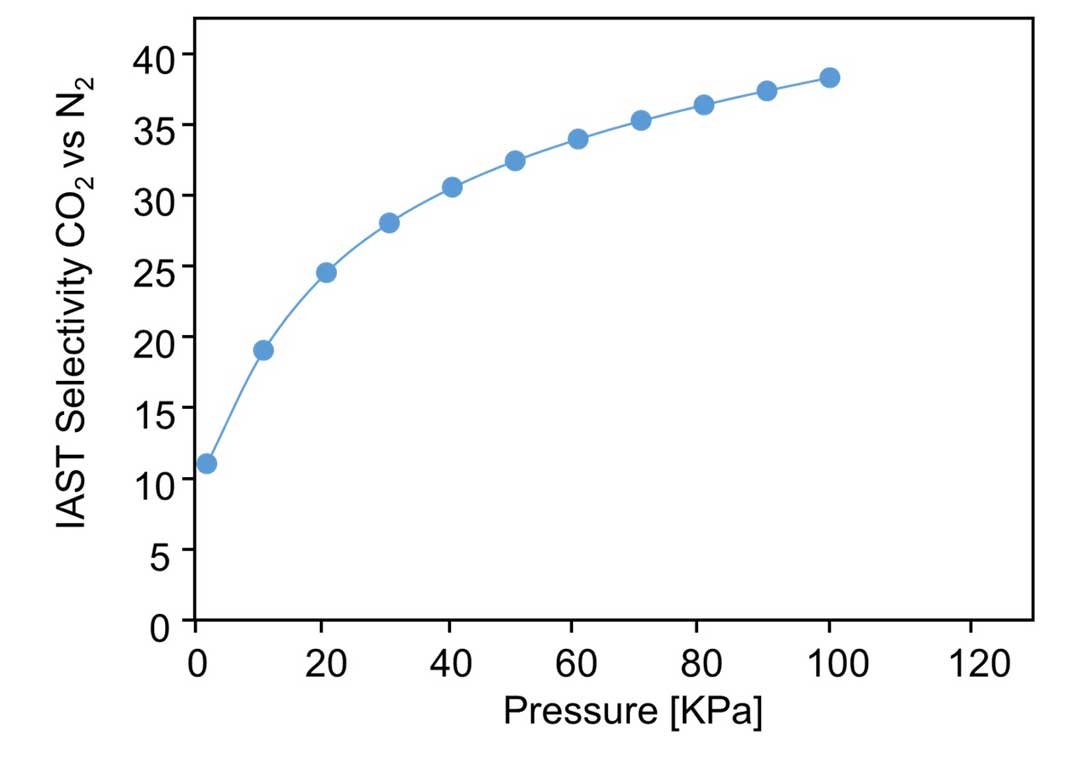
Figure 6: CO2/N2 selectivity at room temperature
4. Social contributions and ripple effects
5. Future developments
In this future-themed project, a single research group selected and synthesized a target molecule, synthesized an MOF, evaluated its gas adsorption abilities, and studied its molecular design by analyzing the MOF's crystal structure. This framework allowed the group's members to make rapid decisions in the course of the study. Further industry-academia partnerships are planned to create environmentally friendly molecules and apply them to the difficult problem of hydrogen storage, which will give back to society by contributing to the development of clean energy.
6. References
7. Paper information
Structural Analysis of and Selective CO2 Adsorption in Mixed-Ligand Hydroxamate-based Metal-organic Frameworks
Authors
Koh Sugamata, Chikaze Takagi, Awano Keiko, Teruyuki Iihama, and Mao Minoura
Journal
Dalton Transactions (Royal Society of Chemistry) 2020, 49, 9948-9952.