Oct 09, 2020PRESS RELEASE
Cutting and repairing damaged DNA
Understanding the DNA repair mechanism in plant mitochondria and chloroplasts
Keyword:RESEARCH
OBJECTIVE.
A research group that included Assistant Professor Yusuke Kobayashi of the Ibaraki University Graduate School of Science and Engineering, Professor Yasuhiko Sekine and Assistant Professor Masaki Odahara (now at RIKEN) of the Rikkyo University College of Science, and other researchers from the National Institute of Genetics, RIKEN, Kyoto University, and the National Institute of Advanced Industrial Science and Technology have uncovered part of the mechanism for repairing damaged mitochondrial and chloroplast DNA in plants.
Mitochondrial and chloroplast DNA encode genes that are important for respiration and photosynthesis. Therefore, accumulation of damage in these organelle DNA impairs respiration and photosynthesis activity, thereby leading to plant death. However, until now little has been known about how this damage is repaired.
In this study, the research group revealed that the enzyme MOC1 is involved in homologous recombination, which is a common mechanism for repairing the DNA in plant mitochondria and chloroplasts.
These findings were published on September 25, 2020, on the website of "Plant Physiology," the journal the American Society of Plant Biologists, and will later be officially published in the journal.
Background
Homologous recombination is a common mechanism for repairing damaged DNA. In homologous recombination, damaged DNA is repaired using DNA with the same information (homologous DNA) as a template. Homologous recombination begins when a recombination enzyme called RECA forms a recombination intermediate called a Holliday junction, which is then cleaved by another enzyme (Figure 1A).
The research group recently discovered MOC1, a Holliday junction resolvase only found in the chloroplasts of eukaryotic algae and plants, from an analysis of mutants with an uneven distribution of chloroplast DNA. However, it remained unclear whether MOC1 is involved in the homologous recombination of chloroplast DNA. Further, although a mitochondrial Holliday junction resolvase (CCE1) has been found in yeast, none have been identified in any other organisms, and the mechanism of Holliday junction resolution in plant mitochondria has remained enigmatic.
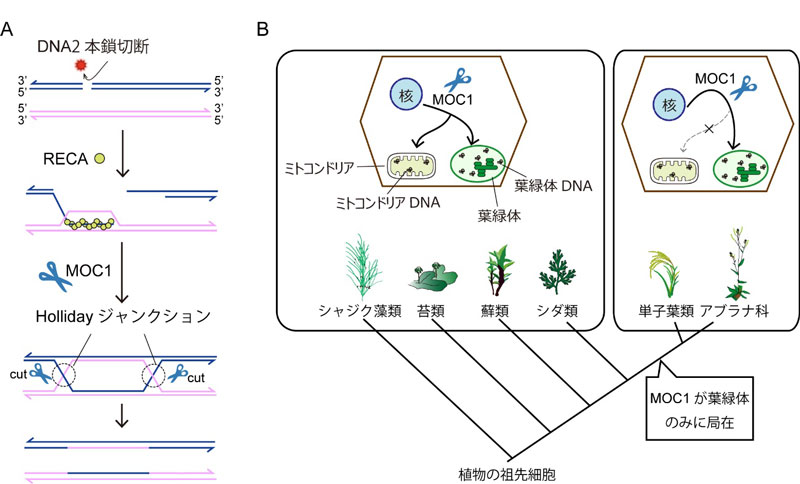
Figure 1. DNA repair mechanism of plant mitochondria and chloroplasts, and its evolution
B: Evolution of the homologous recombination mechanism in plant mitochondria and chloroplasts suggested by the findings of this study. In plant species that diverged in the early stages of land plant evolution, MOC1 was thought to be essential to homologous recombination both in mitochondria and chloroplasts, but plant species that do not require MOC1 for homologous recombination in mitochondria may have appeared. On the other hand, MOC1 is found in chloroplasts from algae to plants, suggesting that the mechanism of Holliday junction resolution is highly conserved in chloroplasts.
Results and methods
Next, the group focused on the fact that MOC1 is widely conserved in algae and plants, and examined whether MOC1 also functions in mitochondria. Predictions using programs and experiments suggested that MOC1 is found in mitochondria as well as chloroplasts in Charales, liverworts, mosses, ferns, and some spermatophytes. The group investigated the involvement of MOC1 in mitochondrial homologous recombination using Physcomitrella patens, a moss with MOC1 localizing both in chloroplasts and mitochondria. The investigation revealed that mutations were highly accumulated in both chloroplast and mitochondrial DNA in P. patens MOC1 gene mutants, which significantly inhibited plant growth.
These findings suggest that MOC1 contributes to the stability of chloroplast DNA in many algal and plant species, and showed that MOC1 is essential for DNA repair in both mitochondria and chloroplasts in bryophytes. Furthermore, due to the presence of MOC1 in the mitochondria of Charales, ferns, and some spermatophytes, MOC1 is expected to be involved in homologous recombination in both chloroplasts and mitochondria in these plant species. In contrast, some plant species do not have MOC1 in mitochondria, which demonstrates the diversity of the mechanisms of mitochondrial homologous recombination in plants (Figure 1B).
Future prospects
Article information
Authors: Yusuke Kobayashi, Masaki Odahara, Yasuhiko Sekine, Takashi Hamaji, Sumire Fujiwara, Yoshiki Nishimura, Shin-ya Miyagishima
Journal: Plant Physiology
Release date: September 25, 2020, online version
DOI: 10.1104/pp.20.00763
* This study was funded by grants including a government grant-in-aid for scientific research, Japan Society for the Promotion of Science grant (18J01993), young researcher grant (20K15812), and basic research A grant (17H01446).