May 14, 2020PRESS RELEASE
"Catalytic asymmetric iodoesterification"—the world’s first!
From Chiba to the world, offering a foundation for the creation of functional iodine compounds
Keyword:RESEARCH
OBJECTIVE.
Through a joint project participated by the laboratory of Professor Takayoshi Arai (Director, Soft Molecular Activation Research Center and Chiba Iodine Resource Innovation Center) at Chiba University Graduate School of Science and the laboratory of Professor Masahiro Yamanaka at Rikkyo University College of Science, the synthesis of iodoesters was successfully carried out for the first time in the world through a catalytic asymmetric iodoesterification of simple alkenes and carboxylic acids—an achievement that was brought about by combining unique catalytic reaction control technology and theoretical calculations of chemical reactions (Figure 1). This reaction will allow for the production of high-performance materials using inexpensive and easily available starting compounds. Moreover, this chemical synthesis method is expected to contribute to the diversification of drug functions through applications in the pharmaceutical field.
The results of their study were published in the German chemistry journal "Angewandte Chemie International Edition."
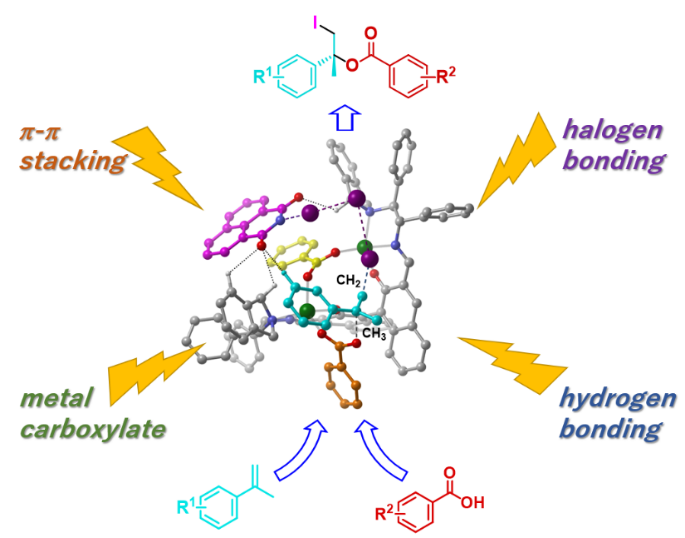
Figure 1: Conceptual diagram of catalytic asymmetric iodoesterification. Four chemical interactions—metal carboxylate formation, halogen bonding, hydrogen bonding, and π-π stacking—harmonized on a single catalytic system enabled them to successfully achieve this synthetic reaction for the first time in the world.
Study background and objective
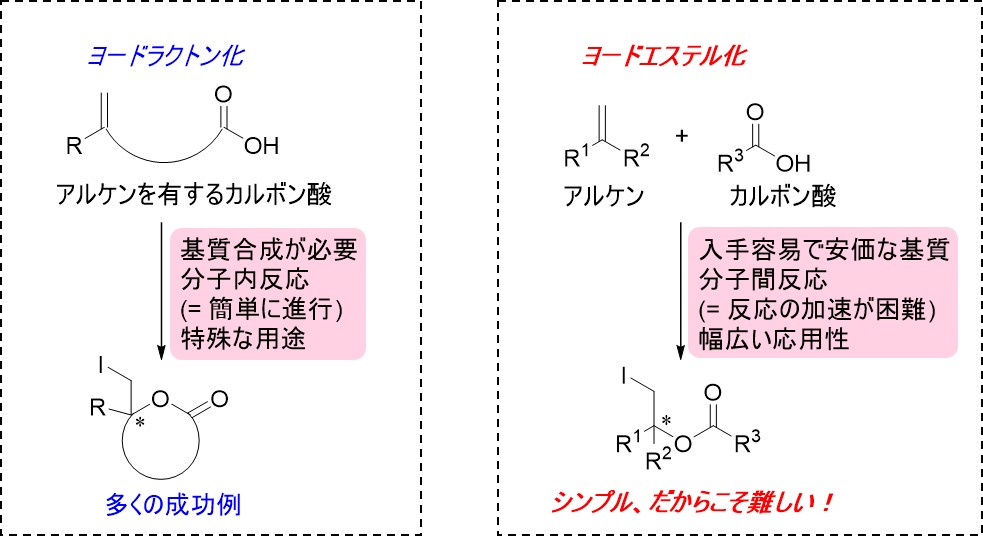
Figure 2: Iodolactonization and iodoesterification. Both are haloesterification reactions using iodine, but in the iodoesterification reaction, the intermolecular reaction of different molecules, i.e., an alkene and a carboxylic acid, needs to be promoted, and this requires advanced stereostructural recognition and control, which are extremely difficult to achieve. Thus, there have been no successful reactions until now.
Esters obtained from carboxylic acids and alcohols are important compounds that are widely used in industry, such as in the manufacture of chemicals and pharmaceuticals. In particular, “catalytic asymmetric iodoesterification,” which is one of the synthetic reactions of ester compounds using iodine, has very high industrial value because it simplifies industrial processes; the synthesis can be carried out using inexpensive and commercially available starting compounds, as compared to the widely used iodolactonization reaction. However, unlike iodolactonization, which is based on intramolecular reactions, catalytic asymmetric iodoesterification requires promoting reactions between different molecules, and to achieve this, advanced molecular structural recognition, such as stereoselectivity, as well as its precise control is essential. Thus, no one has been able to complete the synthesis until now. (Figure 2)
Study findings
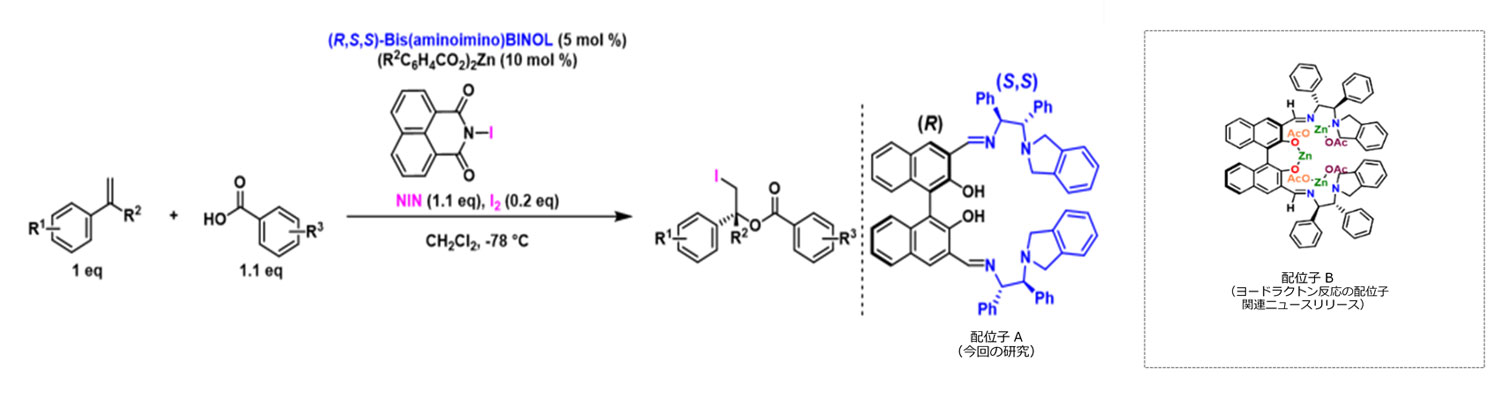
Figure 3: Catalytic asymmetric iodoesterification reaction and its ligands, demonstrated successfully in this study for the first time in the world, and the structural formula of the asymmetric iodolactone catalyst that was previously elucidated. Ligands A and B are structural isomers.
As a result, ligand A (stereoisomer) having a different three-dimensional structure from that in the iodolactonization reaction was developed, and the optimal conditions for obtaining iodoesters were successfully identified. Moreover, by using this new reaction, the desired iodoesters were obtained in high chemical yields with high enantiomeric excess Note 1) (Figure 3).
In addition, detailed analysis of the mechanism of iodoester formation in this catalytic asymmetric reaction revealed that, as illustrated in Figure 1, a total of four different forces, including metal carboxylate formation, halogen bonding Note 2), hydrogen bonding, and π-π stacking Note 3), were harmonized on a single catalytic system, enabling a successful reaction. Among the four forces, metal carboxylate formation was the strongest and most dominant force, and how best to arrange and harmonize the remaining three forces in appropriate positions became an issue. In the present study, in order to enhance the π-π stacking force, a new iodination agent having a highly planar naphthalene skeleton was developed and used in the reaction, thereby achieving a high enantiomeric excess.
【Related News Release】
Social contribution, ramifications
Comments by researcher (Takayoshi Arai, Director of the Soft Molecular Activation Research Center, Director of Chiba Iodine Resource Innovation Center)
Article Information
Journal name: Angewandte Chemie International Edition
DOI:https://doi.org/10.1002/anie.202003886
About the research project
- Grant-in-Aid for Scientific Research (B) basic research JP19H02709
- Grants-in-Aid for Scientific Research (JP16H01004, JP18H04237, JP17KT0011)
Terminology
1) Enantiomeric excess (ee)
For a chiral compound, when one enantiomer is represented by R and the other by S, with the molecular weight of each enantiomer being [R] and [S], the enantiomeric excess of the compound can be defined by the following equation.
2) Halogen bonding:
A non-covalent electrostatic interaction between a halogen atom (Lewis acid), such as iodine, and a Lewis base. As a new interaction with clear directivity, its application in catalytic chemistry and functional molecule creation is anticipated.
3) π-π stacking:
A dispersion force that acts between the aromatic rings of organic molecules. Two aromatic rings are stabilized in an arrangement that resembles stacked coins.