Dec 06, 2023PRESS RELEASE
Success in developing a novel environmentally friendly porous material capable of storing large amounts of hydrogen and carbon dioxide
Keyword:INFORMATION
OBJECTIVE.
The “Mirai Theme Project” laboratory, as part of a Rikkyo University research project* endowed by Nippon Soda Co., Ltd.**, has succeeded in developing Trp-MOF, materials capable of adsorbing large amounts of hydrogen and the greenhouse gas carbon dioxide (Figure 1). The research group, whose members include Rikkyo University Department of Chemistry Professor Mao Minoura, Specially Appointed Associate Professor Koh Sugamata, Visiting Professor Akihiro Shirai, and Visiting Professor Natsuki Amanokura, has been researching novel environmentally friendly molecules. This material could be used in small “molecular cylinders” potentially capable of replacing typical large gas cylinders. This discovery is the fruit of industry-academia collaboration between Nippon Soda and Rikkyo University and was published to wide acclaim in the academic journal “Chemistry - A European Journal,” with the molecule featured on the cover.
Rikkyo University established a system of endowed research projects with the hope of facilitating collaboration between industry and academia. Since 2017, the university has carried out projects endowed by corporations with the goal of contributing to society by advancing and revitalizing research and education and by collaborating on technological issues.
**) Nippon Soda Co., Ltd.
A general chemical company (headed by President Eiji Aga) that celebrated its 100th anniversary in 2020. Under its new slogan “Brilliance through chemistry,” Nippon Soda manufactures and markets high value-added chemical products for industries such as agriculture, pharmaceuticals, and electronics.
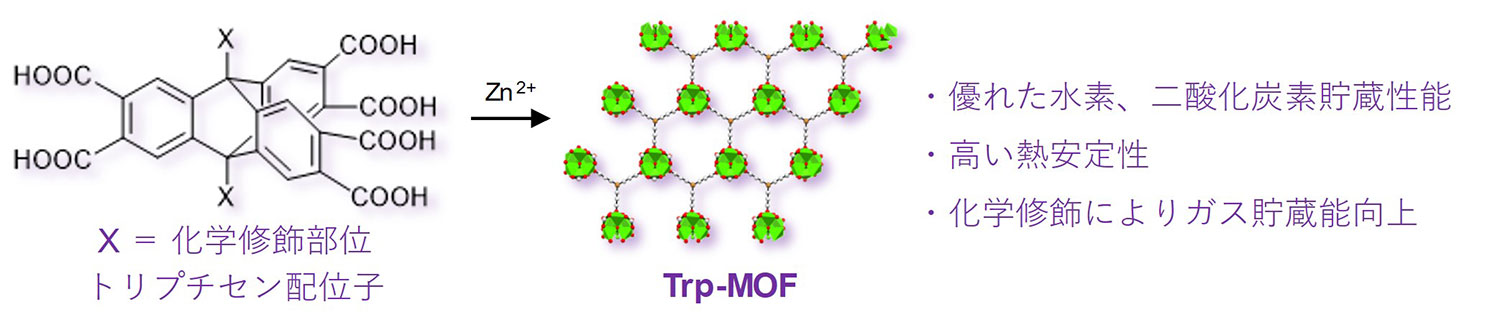
Figure 1. Overview of the study
1. Main findings
Efficiently storing gas in regularly arranged hexagonal “honeycombs”
2) Trp-MOFs, characterized by their honeycomb-like structure, exhibit an extraordinary capacity for the efficient storage of carbon dioxide and hydrogen.
3) The material demonstrated impressive thermal stability, remaining undecomposed even at temperatures exceeding 400 °C.
Metal-organic frameworks (MOFs)*1) have extremely small pores called micropores*2) that have much higher specific surface area*3) than conventional porous materials such as activated carbon and zeolite*4). This feature raises the possibility of using MOFs for gas adsorption and separation. The research group synthesized triptycene-2,3,6,7,14,15-hexacarboxylic acid and its derivatives as organic ligands and reacted them with zinc nitrate to create an MOFs with excellent carbon dioxide and hydrogen storage capacity (Figure 1). The use of a rigid and thermally stable molecule called triptycene as the organic ligand endowed the MOFs with impressive thermal stability. Further, functionalization of the triptycene ligand with an alkyl group or halogen further improved gas adsorption.
2. Study background
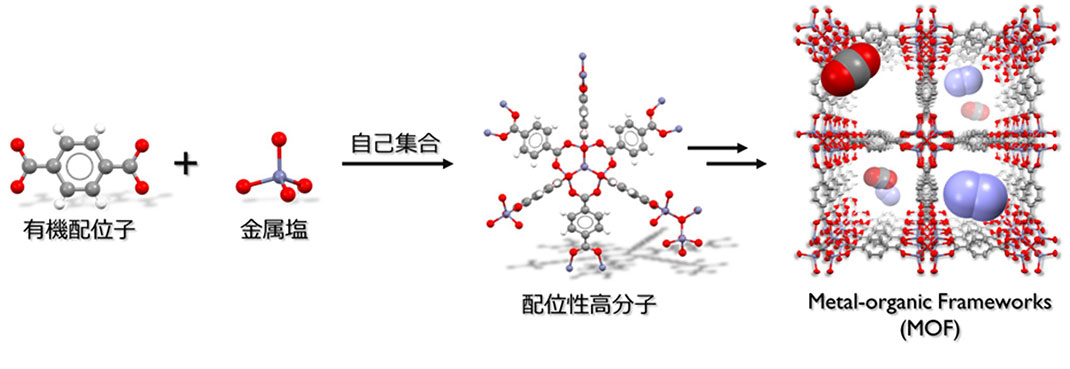
Figure 2. Synthesis and structure of metal-organic frameworks
Porous metal-organic frameworks (MOFs) have attracted much attention recently for their potential role as highly efficient adsorbent materials for these gases (Figure 2). MOFs have sponge-like properties that allow them to physically adsorb gases, allowing them to easily adsorb or desorb gases simply by changing the pressure or temperature of the environment. Thus, it is hoped they could be used as separation membranes or safe gas storage materials.
3. Study findings
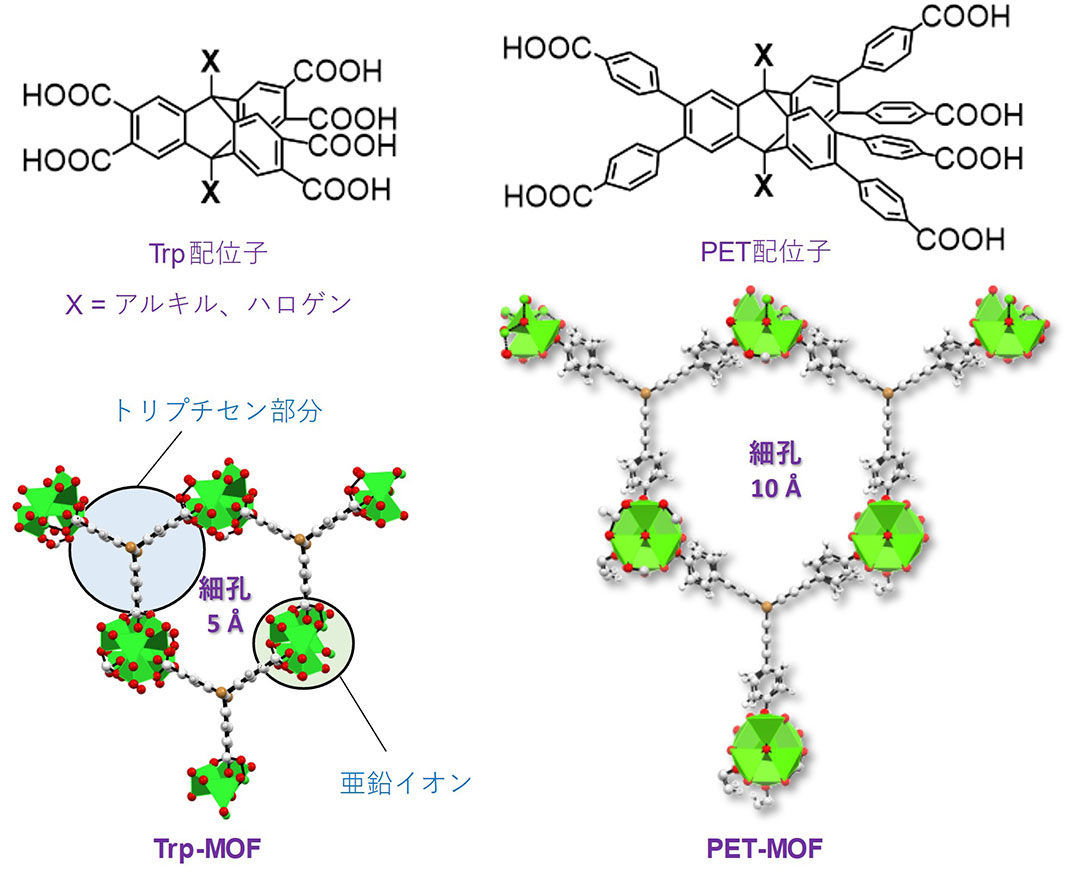
Figure 3. Structure of triptycene ligand and MOF
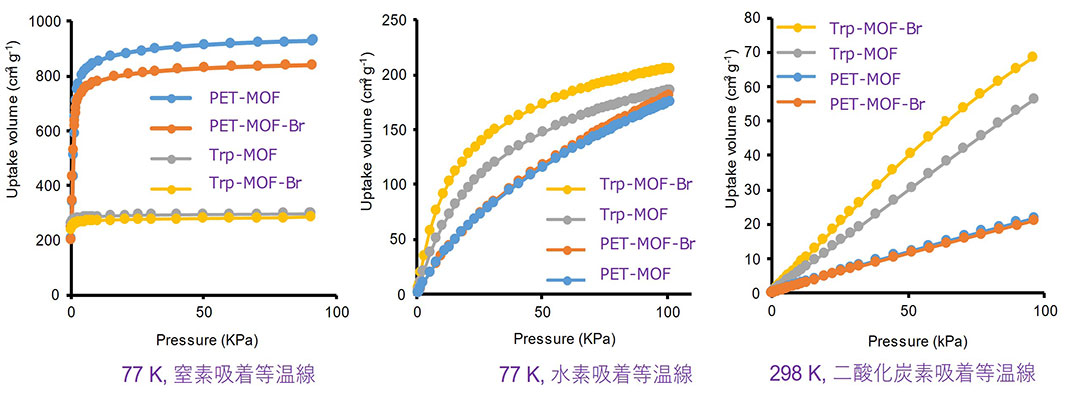
Figure 4. Nitrogen, hydrogen, carbon dioxide adsorption experiments
Carbon dioxide adsorption experiments at room temperature were also conducted on each of the MOFs. Trp-MOF-Br and Trp-MOF both exhibited excellent carbon dioxide adsorption, about 3 times higher than PET-MOF, which has larger pores. Further, because of the excellent thermal stability of triptycene, both Trp-MOF and PET-MOF had extremely high thermal stability, remaining undecomposed even at 400 °C, demonstrating that these MOFs could be used even under harsh conditions.
4. Social contribution, ripple effects
5. Future developments
As part of the “Mirai Theme Research Project,” an industry-academia partnership under Rikkyo University's Faculty of Science, the research group selected and synthesized target molecules, synthesized MOFs, evaluated their gas adsorption abilities, and verified the MOFs molecular design through crystal structure analysis. The project structure allowed for rapid decision-making, implementation, verification, and feedback. The aim of this industry-academia partnership is to create environmentally friendly molecules, to use the power of chemistry to develop materials to resolve the difficult problem of storing substances such as hydrogen, and to put these findings into practice in society as part of the shift to clean energy.
6. Glossary
*2) Micropores: A type of tiny pore that exists in porous materials with a diameter of 2 nm or less. Pores that are 2-50 nm are called mesopores, and those 50 nm or larger are called macropores.
*3) Specific surface area: The surface area (m2) per unit mass (g) of a substance.
*4) Zeolite: A general term for crystalline aluminosilicate. The substance was discovered in nature and has since been synthesized with various pore sizes and shapes.
7. Article information
Zn-based Metal-Organic Frameworks Using Triptycene Hexacarboxylate Ligands: Synthesis, Structure, and Gas-Sorption Properties
Authors:
Koh Sugamata, Shoko Yamada, Daichi Yanagisawa, Natsuki Amanokura, Akihiro Shirai, and Mao Minoura
Journal:
Chemistry - A European Journal
DOI: