Nov 21, 2019PRESS RELEASE
Successful activation of π electron system using halogen bond-donor iodine catalyst -- A novel method of synthesizing useful indole derivatives for drug development and other applications
Keyword:RESEARCH
OBJECTIVE.
Prof. Takayoshi Arai (director of the Soft Molecular Activation Research Center and the Chiba Iodine Resource Innovation Center) and Assistant Prof. Satoru Kuwano (Molecular Chirality Research Center) of the Chiba University Graduate School of Science, Faculty of Science, have succededed in "developing π electron compound activation functions using a halogen bond-donor iodine catalyst" (Figure 1). This study was the result of joint research with Prof. Masahiro Yamanaka of the Rikkyo University Department of Chemistry.
Research overview
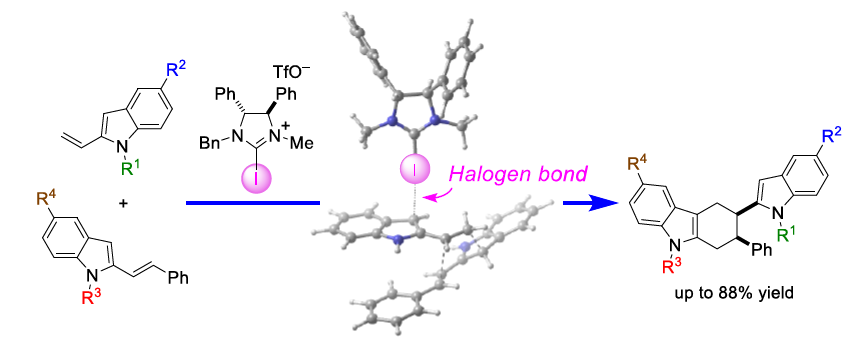
Figure 1: Synthesis of indole derivatives based on activation of π electron system compounds using a halogen bond-donor iodine catalyst
Study background and objective
Results
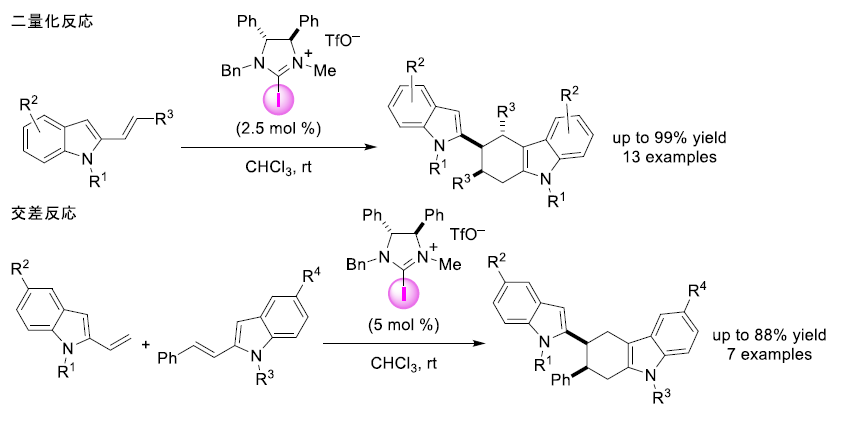
Figure 2. [4+2] cycloaddition reaction using a halogen bond-donor iodine catalyst
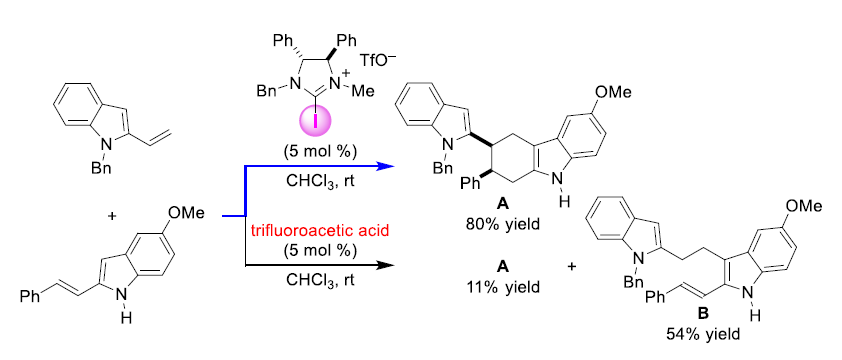
Figure 3: Comparison of iodine catalyst and Brønsted acid catalyst
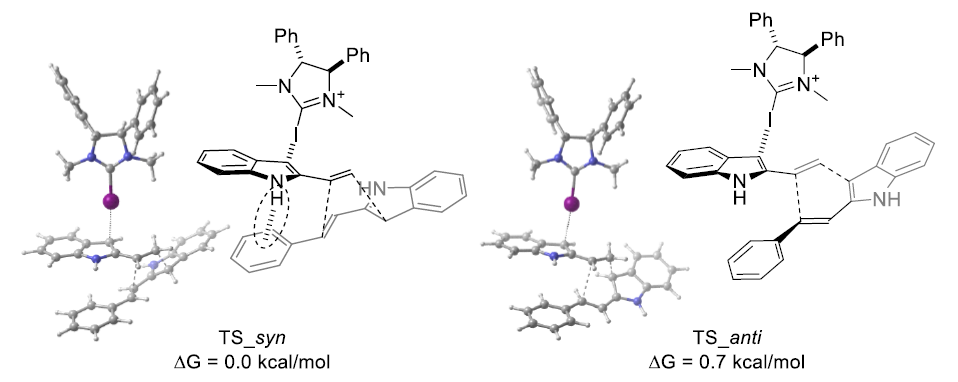
Figure 4: Analysis of transition states mediated by CI···π halogen bonds
Originality
Social contributions, ramifications
The results were published in Angewandte Chemie International Edition, a top chemistry journal.
Kuwano, S.; Suzuki, T.; Yamanaka, M.; Tsutsumi, R.; Arai, T. Angew. Chem. Int.Ed., [10.1002/ange.201904689].
This study was funded by the following grant-in-aids for scientific research: basic research (B) JP19H02709, young researchers 19K15553, and research in new academic fields JP16H01004 and JP18H04237 (precision controlled reactions) and JP18H04660 (hybrid catalysts). Assistance was also provided by the Futaba Foundation.