Nov 21, 2019PRESS RELEASE
Development of a novel method for synthesizing pharmaceutical compounds containing fluorine atoms using sequential organic synthesis reactions
Keyword:RESEARCH
OBJECTIVE.
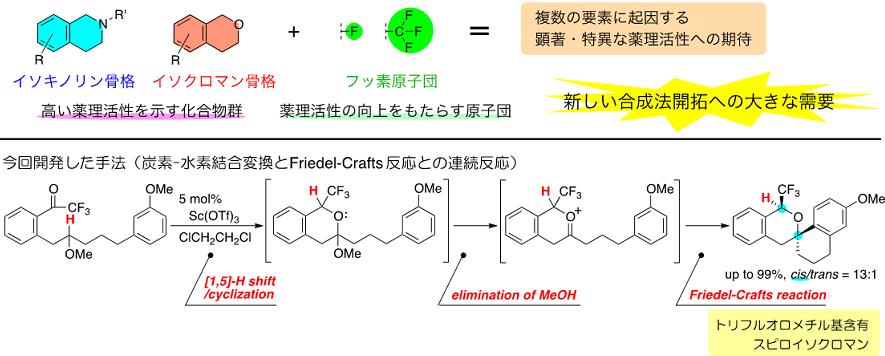
Associate Professor Keiji Mori of Tokyo University of Agriculture and Technology's Graduate School of Engineering, Department of Applied Chemistry; Professor Akihiko Takayama of Gakushuin University's Department of Chemistry; and Professor Masahiro Yamanaka of Rikkyo University's Department of Chemistry succeeded in the stereoselective synthesis of polycyclic compounds (spiroisochromans) with a trifluoromethyl group (-CF3) by combining carbon-hydrogen bond transformation, which is a difficult chemical reaction to generate, with a basic organic reaction. Their result is expected to open up pathways for supplying new drug candidate compounds that could not be synthesized using previous methods.
Current state
Research structure
Reaserch results
Future prospects