Feb 06, 2019
Short-step synthesis of chiral compound using carbon-hydrogen bond double conversion
Keyword:RESEARCH
OBJECTIVE.
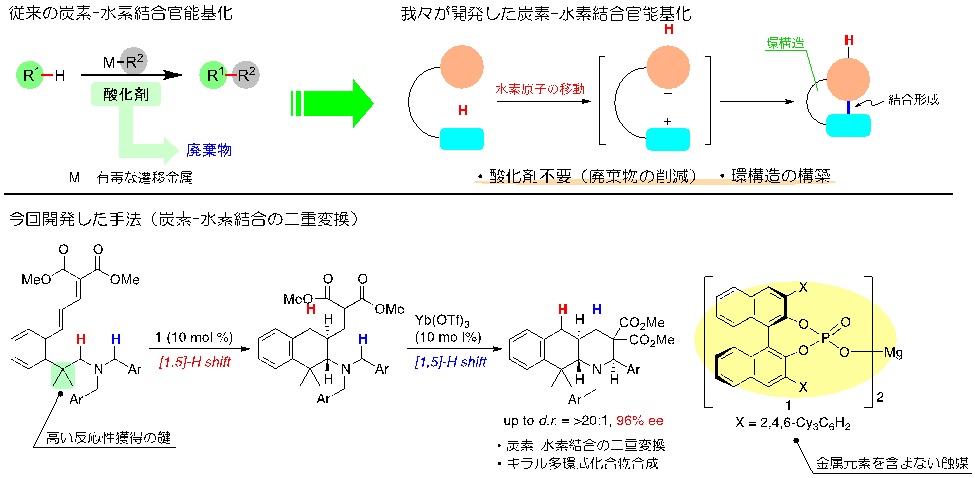
Associate Professor Keiji Mori of the Department of Applied Chemistry, Graduate School of Engineering, Tokyo University of Agriculture and Technology; Professor Takahiko Akiyama of the Department of Chemistry, Faculty of Science, Gakushuin University; and Professor Masahiro Yamanaka of the Department of Chemistry and Research Center for Smart Molecules, Rikkyo University developed a method for short-step synthesis of a polycyclic compound through sequential stereoselective conversion of carbon-hydrogen bonds, which are incredibly difficult to chemically react. These results could open the door to compounds with structures that previously could not be synthesized to provide an efficient supply of candidate compounds for new pharmaceuticals.
Article title: Chiral Magnesium Bisphosphate Catalyzed Asymmetric Double C(sp3)–H Bond Functionalization Based on Sequential Hydride Shift/Cyclization Process
URL:https://pubs.acs.org/doi/10.1021/jacs.8b02761
Present circumstances
A variety of direct conversion methods have already been developed, but these have either required expensive and toxic transition metal catalysts or an oxidizing agent that then becomes a waste product. There have been only a few successful examples of sequential double carbon-hydrogen bond activation that were able to synthesize complex compounds such as drug candidate compounds in a short-step synthesis.
Study organization
Results
Future prospects
1) Chiral: Cannot be superpositioned on its mirror image.
2) Asymmetric synthesis: To selectively synthesize 1 side of an enantiomer.
Other News
-
Sep 27, 2023
PRESS RELEASE